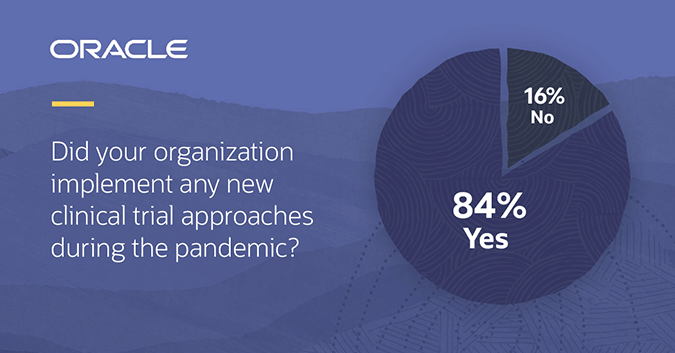
COVID-19 physical distancing requirements forced change in how traditional clinical trials were conducted and a new Informa Pharma and Oracle study shows the industry is not looking back. Of the 251 people surveyed, 84% implemented alternative tactics to continue their existing clinical trials and/or start new trials during the pandemic. Of those respondents, 82% said these approaches had positively impacted their clinical trials overall. Looking to the future, 97% indicated their organization would continue using at least one of these new methods moving forward, leading with remote monitoring and video (eHealth) visits.
“At the onset of the pandemic, the industry had to quickly adapt to keep trials afloat and this forced change helped researchers understand when and how to implement these approaches to improve clinical research,” said Henry McNamara, senior vice president and general manager, Oracle Health Sciences. “Methods and technology that were being explored pre-pandemic have come front and center during the past 18 months. As clinical trials continue to evolve, study teams can rest assured that technology will help carry them into the future and speed new discoveries.”
From our partners:
The survey, Clinical Trial Management in a Post-Pandemic World, polled participants from biopharmaceutical and medical device companies, as well as CROs involved in the operations and management of clinical trials from July to September 2021. Respondents, who were largely from North America and Europe, with others from APAC and other regions, were asked about changes made to their clinical trials during the pandemic, the effect of these adaptations, and the impact of these changes on the future of clinical trials.
Newly implemented trial methods bring benefits to trials and patients
Of the various methods noted, 45% of respondents said they had adopted remote monitoring in their trials during the pandemic, nearly doubling the number of respondents who reported using remote monitoring a year ago. Among the newly adopted methods that companies plan to continue to use post-pandemic, remote monitoring (32%), video visits (28%), electronic health records (EHR) (24%), and phone visits led the pack (24%).
The most common positive impacts of the alternative approaches adopted during the pandemic included more timely data access (48%), improved flexibility for patients (41%), and increased speed (38%).
When asked about their confidence in the data generated by newly adopted approaches, 92% of respondents stated they were equally or more confident in the data collected from these methods, compared to data collected via pre-pandemic methods. This was a marked change from the 2020 survey when nearly half of respondents said that data reliability and quality (50%) and data collection (45%) were key concerns in adopting decentralized clinical trials. This significant increase in confidence supports the industry’s continued progress towards more decentralized models.
The study also found that improving the patient experience is a priority. Many respondents (61%) believe allowing patients’ choice will have a positive impact on clinical research and well over half (58%) said that their organizations plan to give patients the option to choose how they participate in clinical trials moving forward.
As the shift to decentralized trials continues, survey respondents expect their organizations to continue using hybrid (44%) and fit-for-purpose (42%) models after the pandemic. Of the four models considered—site-based, fit-for-purpose, hybrid, and decentralized—respondents expect the use of the site-based model to decrease the most (24%).
“The COVID-19 pandemic challenged status quo and served as a catalyst for the adoption of the technology-enabled ‘patient-centric’ decentralized clinical trials model,” said Dr. Nimita Limaye, research vice president, IDC. “As data overflowed from diverse sources, data collection models transitioned from ‘discrete’ data points to ‘continuous’ measures and from ‘local optimums’ to unified cloud-native platforms. The reality of this shift to the new norm has led to most of the industry planning on continuing to implement newer decentralized clinical trial models, with a significant reduction in the traditional site-based model. It is evident that patient optionality rules the day, with most organizations planning on giving patients the choice about how they participate in clinical trials.”
To learn more about the study, join an in-depth webinar on the research and findings on October 26th here or download the full report here. Learn more about Oracle Health Sciences Clinical One here.
About Oracle Health Sciences
As a leader in Life Sciences cloud technology, Oracle Health Sciences’ Clinical One and Safety One are trusted globally by professionals in both large and emerging companies engaged in clinical research and pharmacovigilance. With over 20 years’ experience, Oracle Health Sciences is committed to supporting clinical development, delivering innovation to accelerate advancements, and empowering the Life Sciences industry to improve patient outcomes. Oracle Health Sciences. For life.
About Oracle
Oracle offers integrated suites of applications plus secure, autonomous infrastructure in the Oracle Cloud. For more information about Oracle (NYSE: ORCL), please visit us at www.oracle.com.
Trademarks
Oracle, Java, and MySQL are registered trademarks of Oracle Corporation.
For enquiries, product placements, sponsorships, and collaborations, connect with us at [email protected]. We'd love to hear from you!
Our humans need coffee too! Your support is highly appreciated, thank you!